carboxylic acid ka formula Naming carboxylic acids
Carboxylic acids are an important class of organic compounds that have found applications in various fields. These compounds are known for their acidic properties and are widely used in the production of drugs, flavors, and fragrances. If you are a chemistry student, you may have heard about carboxylic acids and their properties. In this post, we will discuss the general formula of carboxylic acids and their common names. The general formula for carboxylic acids is R-COOH, where R represents an alkyl or aryl group. The -COOH group is a functional group that gives carboxylic acids their characteristic acidic properties. When carboxylic acids are dissolved in water, they ionize to form H+ ions and carboxylate ions (-COO-). These ions can react with other substances to form salts, esters, and acid halides. Some common names of carboxylic acids include formic acid (HCOOH), acetic acid (CH3COOH), propionic acid (CH3CH2COOH), butyric acid (CH3(CH2)2COOH), and benzoic acid (C6H5COOH). These acids are widely used in the production of pharmaceuticals, food additives, and plastics. For example, acetic acid is used in the production of vinegar, while benzoic acid is used as a preservative in foods. To better understand the general formula of carboxylic acids, let’s take a closer look at the structure of acetic acid. Acetic acid has the formula CH3COOH, where CH3 represents a methyl group (-CH3) and COOH represents the -COOH functional group. The -COOH group is attached to the carbon atom adjacent to the carbonyl group (-C=O). The carbonyl group is a characteristic feature of many organic compounds and gives them unique properties. Now that we have discussed the general formula of carboxylic acids and their common names, let’s summarize the key takeaways. Carboxylic acids are organic compounds that have a -COOH functional group and are known for their acidic properties. The general formula for carboxylic acids is R-COOH, where R represents an alkyl or aryl group. Some common names of carboxylic acids include formic acid, acetic acid, propionic acid, butyric acid, and benzoic acid. These acids have found applications in various fields and are used in the production of drugs, flavors, and fragrances. In conclusion, carboxylic acids are an important class of organic compounds that are widely used in various applications. As a chemistry student, it is important to understand the general formula of carboxylic acids and their common names. By gaining a deeper knowledge of these compounds, you can better appreciate their unique properties and applications.
If you are looking for Carboxylic acids - Presentation Chemistry you’ve visit to the right place. We have 5 Images about Carboxylic acids - Presentation Chemistry like What is the general formula of a carboxylic acid class 11 chemistry CBSE, Naming Carboxylic Acids - Chemistry Steps and also Naming Carboxylic Acids - Chemistry Steps. Here you go:
Carboxylic Acids - Presentation Chemistry
.PNG)
What Is The General Formula Of A Carboxylic Acid Class 11 Chemistry CBSE
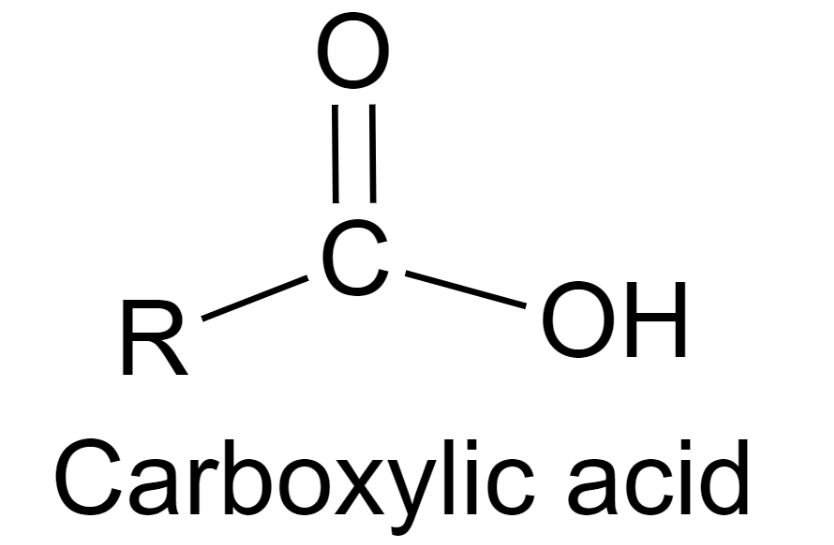 www.vedantu.comcarboxylic acid acidic possess acids
www.vedantu.comcarboxylic acid acidic possess acids
Naming Carboxylic Acids - Chemistry Steps
 www.chemistrysteps.comcarboxylic acids names common naming chemistry these their
www.chemistrysteps.comcarboxylic acids names common naming chemistry these their
A) The General Form Of A Carboxylic Acid, And Examples Of B) A
www.researchgate.netcarboxylic monocarboxylic carboxyl branched straight acids dicarboxylic
Carboxylic Acids - Assignment Point
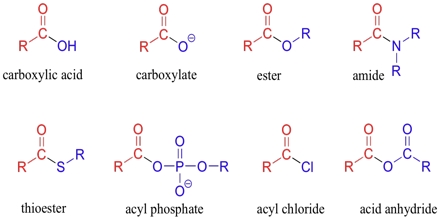 www.assignmentpoint.comcarboxylic acids assignment point lecture assignmentpoint
www.assignmentpoint.comcarboxylic acids assignment point lecture assignmentpoint
Carboxylic acids names common naming chemistry these their. Carboxylic acid acidic possess acids. Carboxylic acids